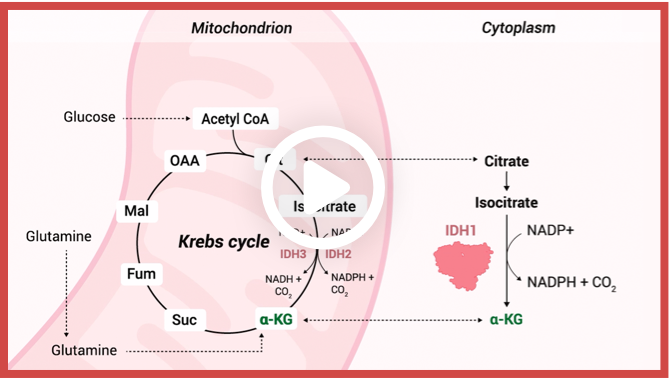
What is IDH?
Overview
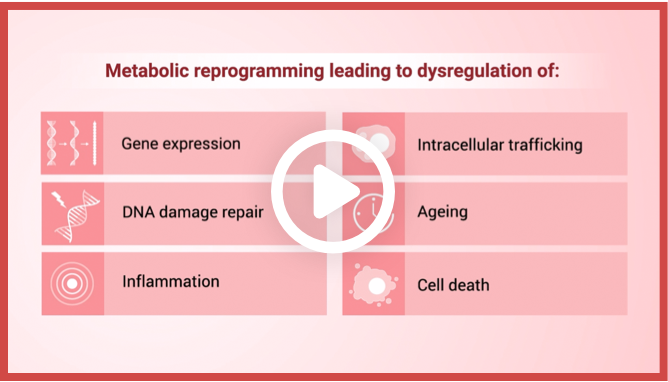
IDH mutations cause metabolic reprogramming that results
in dysregulation of1:

Gene expression

Intracellular trafficking

DNA damage repair

Aging

Inflammation

Cell death
Differences Between IDH1
and IDH2
Differences Between IDH1 and IDH2

Enzymatic reactions catalyzed by wild-type IDH isoforms convert isocitrate to α-KG.2
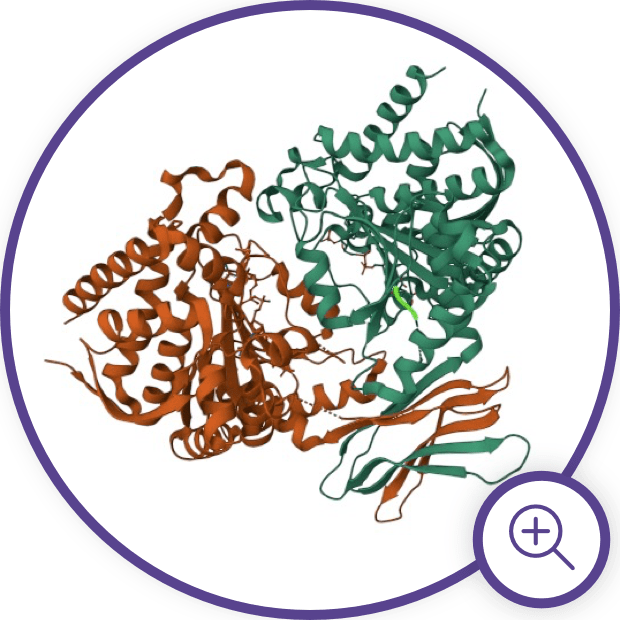
IDH Enzyme Structure and
Gain-of-Function Mutations
mIDH Impact on Cellular
Differentiation via Epigenetic
Changes

Neoplastic activity of mIDH produces high intracellular levels of 2-HG and leads to epigenetic changes that interfere with myeloid differentiation.6,9
mIDH Impact on Immune
System Tumor Response
Additionally, emerging research has unveiled the impact of increased 2-HG levels and its influence on CD8+ T cells in anti-tumor responses.15 2-HG has been found to affect CD8+ T cell function, leading to inhibited proliferation, impaired degranulation, reduced production and release of interferon-ϒ, altered glycolysis, and impaired lactate dehydrogenase (LDH) function within these cells.15 Overall, this impairs the cancer-killing ability of T-cells and allows tumor proliferation.15
IDH mutations are just the tip
of the “iceberg”10–15

Explore video clips on the
function of IDH1 and the
impact of mutation1,2,16–18
Explore video clips on the
function of IDH1 and the
impact of mutation1,2,16–18
Malignancies Associated with
IDH Mutations
Mutations in IDH1 and IDH2 are seen in a variety of cancers, including acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), IDH-mutant glioma, and cholangiocarcinoma, thus reinforcing the key pathogenetic role of these mutations.1
mIDH is considered an early “driver” mutation in MDS and becomes more frequent as the disease progresses to AML.3,4,6,21 Mutations in IDH1 and IDH2 are present in 6% to 16% and 8% to 19% of patients with newly diagnosed AML, respectively.22

Malignancies associated with IDH mutations.1,2,19,20
Stay in the Know
Keep up to date on the latest in
IDH science from leading experts.
References:
1. Pirozzi CJ, Yan H. Nat Rev Clin Oncol. 2021;18(10):645-661. doi:10.1038/s41571-021-00521-0 2. Losman JA, Kaelin WG. Genes Dev. 2013;27(8):836-852. doi:10.1101/gad.217406.113 3. Thol F, Weissinger EM, Krauter J, et al. Haematologica. 2010;95(10):1668-1674. doi:10.3324/haematol.2010.025494 4. Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Leukemia. 2017;31(2):272-281. doi:10.1038/leu.2016.275 5. Jin J, Hu C, Yu M, et al. PLoS ONE. 2014;9(6):e100206. doi:10.1371/journal.pone.0100206 6. Testa U, Castelli G, Pelosi E. Cancers. 2020;12(9):2427. doi:10.3390/cancers12092427 7. Waitkus MS, Diplas BH, Yan H. Cancer Cell. 2018;34(2):186-195. doi:10.1016/j.ccell.2018.04.011 8. RCSB Protein Data Bank website. https://www.rcsb.org/structure/3MAP. Accessed May 18, 2023. 9. Pan D, Rampal R, Mascarenhas J. Blood Advances. 2020;4(5):970-982. doi:10.1182/bloodadvances.2019001245 10. Wang F, Travins J, DeLaBarre B, et al. Science. 2013;340(6132):622-626. doi:10.1126/science.1234769 11. Kernytsky A, Wang F, Hansen E, et al. Blood. 2015;125(2):296-303. doi:10.1182/blood-2013-10-533604 12. Molenaar RJ, Wilmink JW. J Histochem Cytochem. 2022;70(1):83-97. doi:10.1369/00221554211062499 13. Zhao A, Zhou H, Yang J, Li M, Niu T. Sig Transduct Target Ther. 2023;8(1):71. doi:10.1038/s41392-023-01342-6 14. Schvartzman JM, Reuter VP, Koche RP, Thompson CB. Proc Natl Acad Sci USA. 2019;116(26):12851-12856. doi:10.1073/pnas.1817662116 15. Notarangelo G, Spinelli JB, Perez EM, et al. Science. 2022;377(6614):1519-1529. doi:10.1126/science.abj5104 16. Lu C, Ward PS, Kapoor GS, et al. Nature. 2012;483(7390):474-478. doi:10.1038/nature10860 17. Chowdhury R, Yeoh KK, Tian Y, et al. EMBO Reports. 2011;12(5):463-469. doi:10.1038/embor.2011.43 18. Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D. Biomolecules & Therapeutics. 2016;24(1):1-8. doi:10.4062/biomolther.2015.078 19. Urban DJ, Martinez NJ, Davis MI, et al. Sci Rep. 2017;7(1):12758. doi:10.1038/s41598-017-12630-x 20. Mardis ER, Ding L, Dooling DJ, et al. N Engl J Med. 2009;361(11):1058-1066. doi:10.1056/NEJMoa0903840 21. Makishima H, Yoshizato T, Yoshida K, et al. Nat Genet. 2017;49(2):204-212. doi:10.1038/ng.3742 22. DiNardo CD, Ravandi F, Agresta S, et al. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072