Untangling the Role
of IDH in Oncology
Expert Insights on the Molecular Evaluation of AML and MDS

Hematopathologist
Sanam Loghavi, MD, discusses the
significance of driver mutations and
outlines testing strategies in AML

Hematopathologist
Sanam Loghavi, MD, discusses
genetic drivers, risk stratification,
and testing strategies in MDS
What is IDH?
The MDS-AML Spectrum
The Impact of mIDH in
Oncology
The Impact of mIDH in Oncology
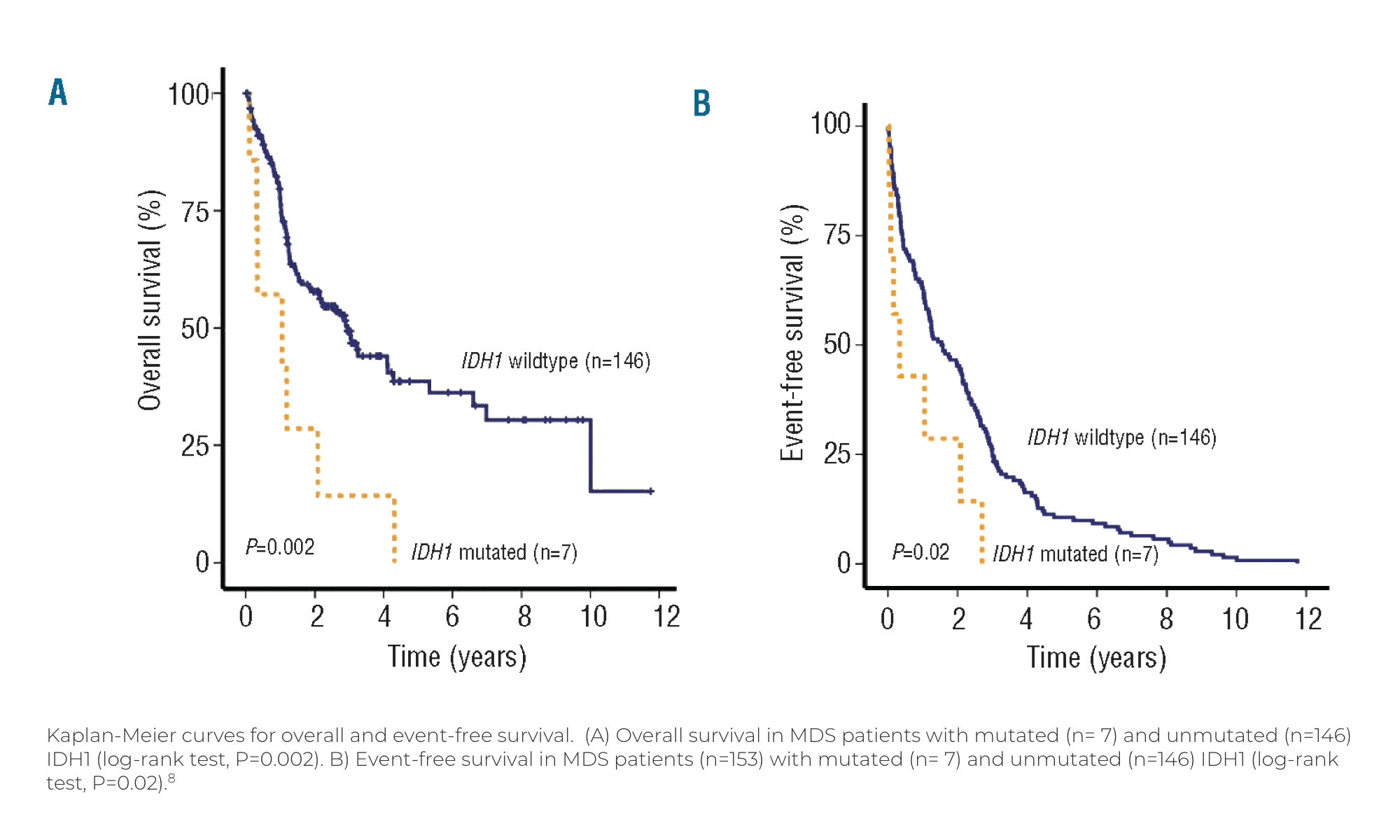
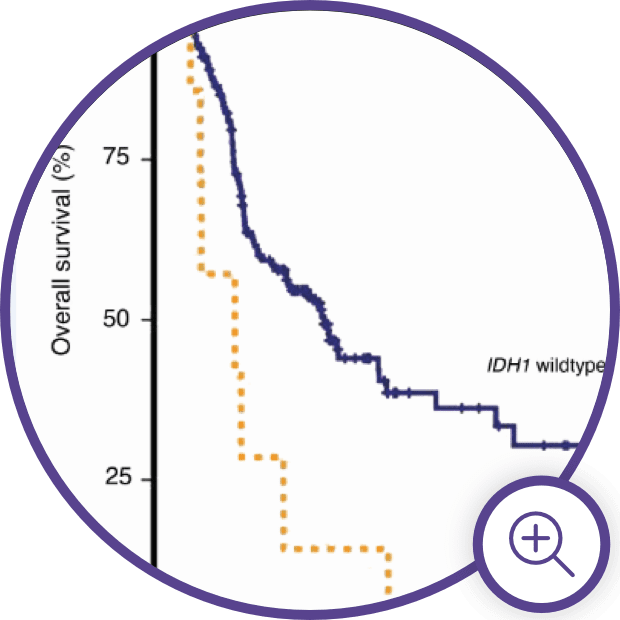
Visualizing the Effect of
IDH Mutations in MDS
Visualizing the Effect of IDH Mutations in MDS
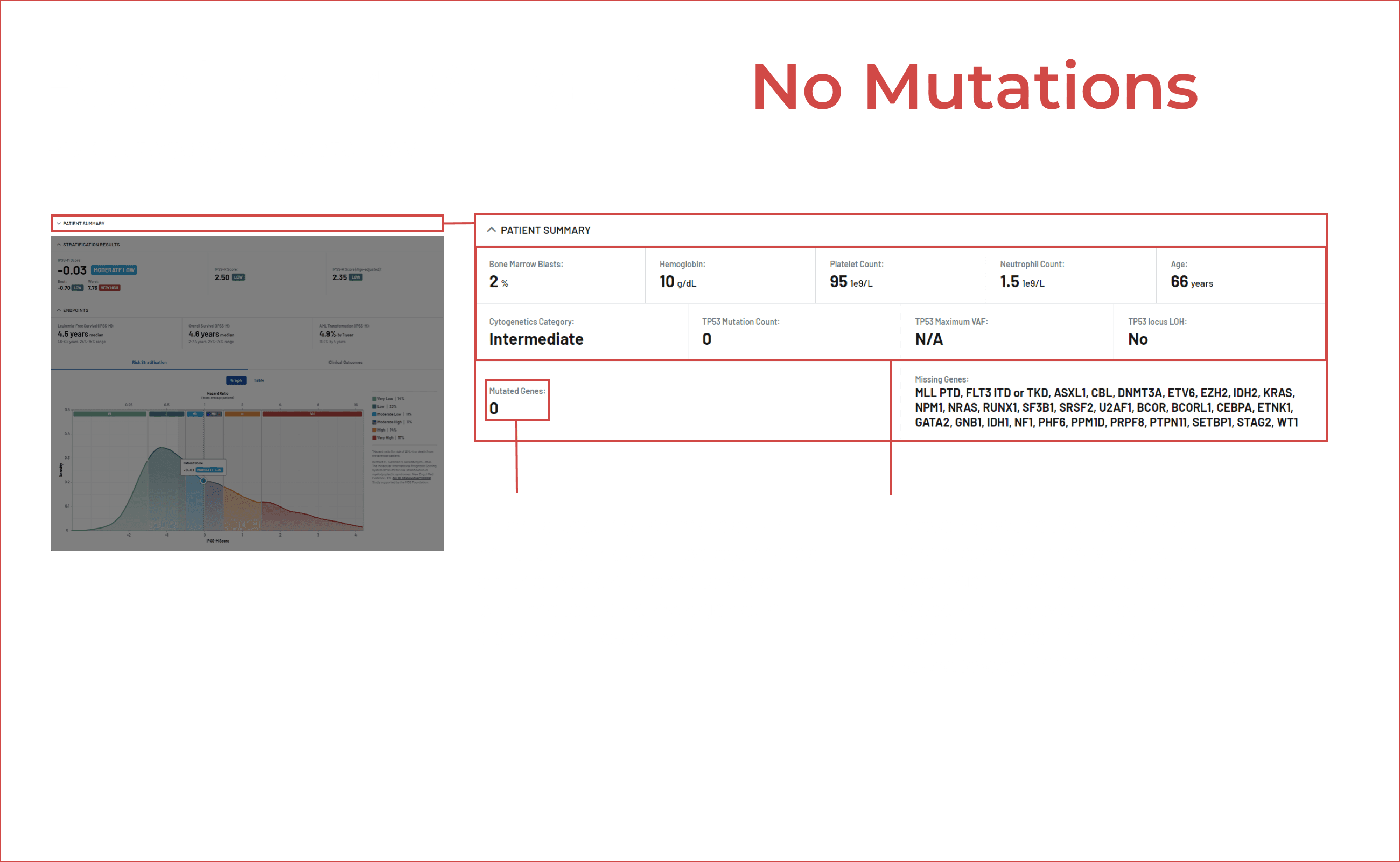
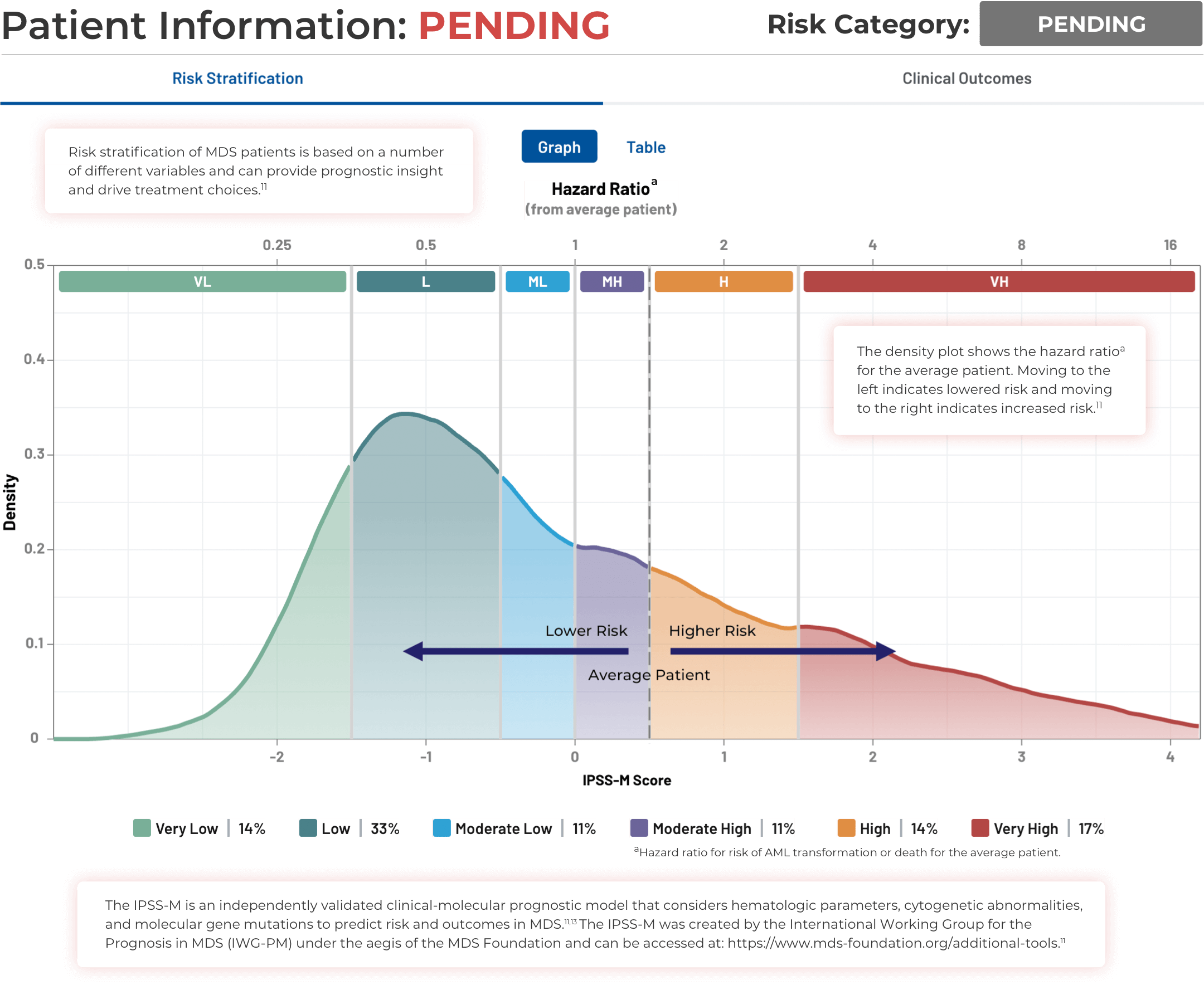
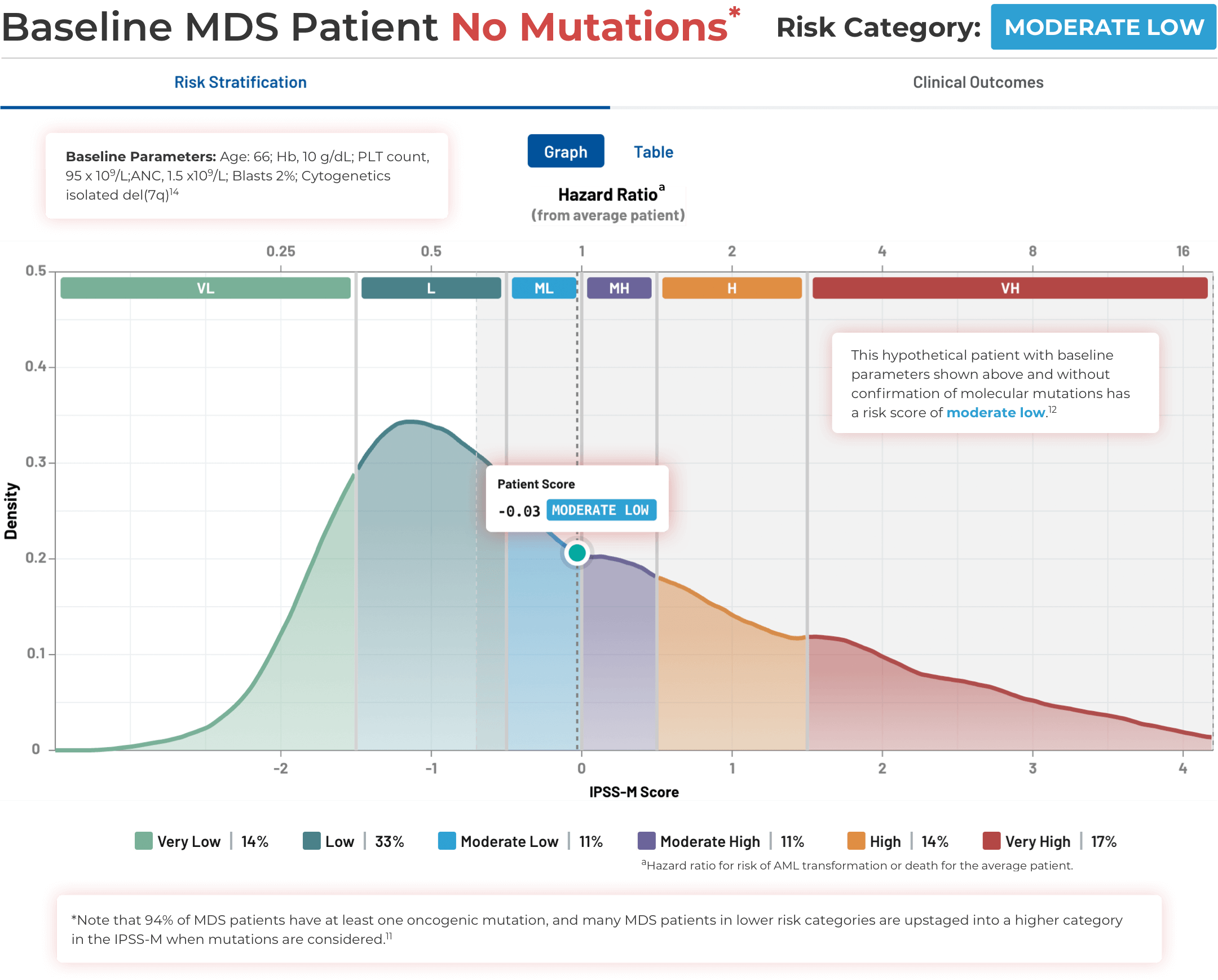
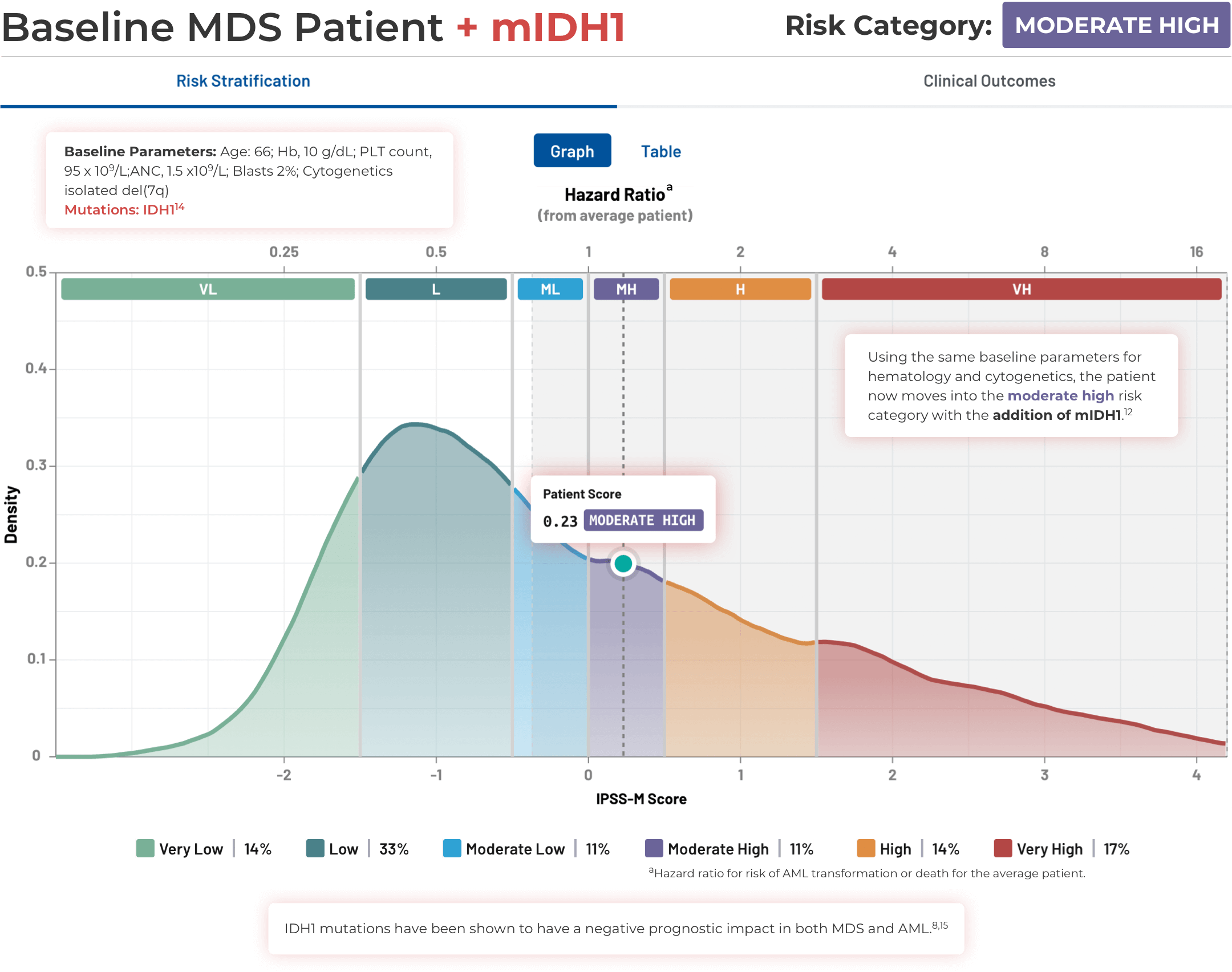
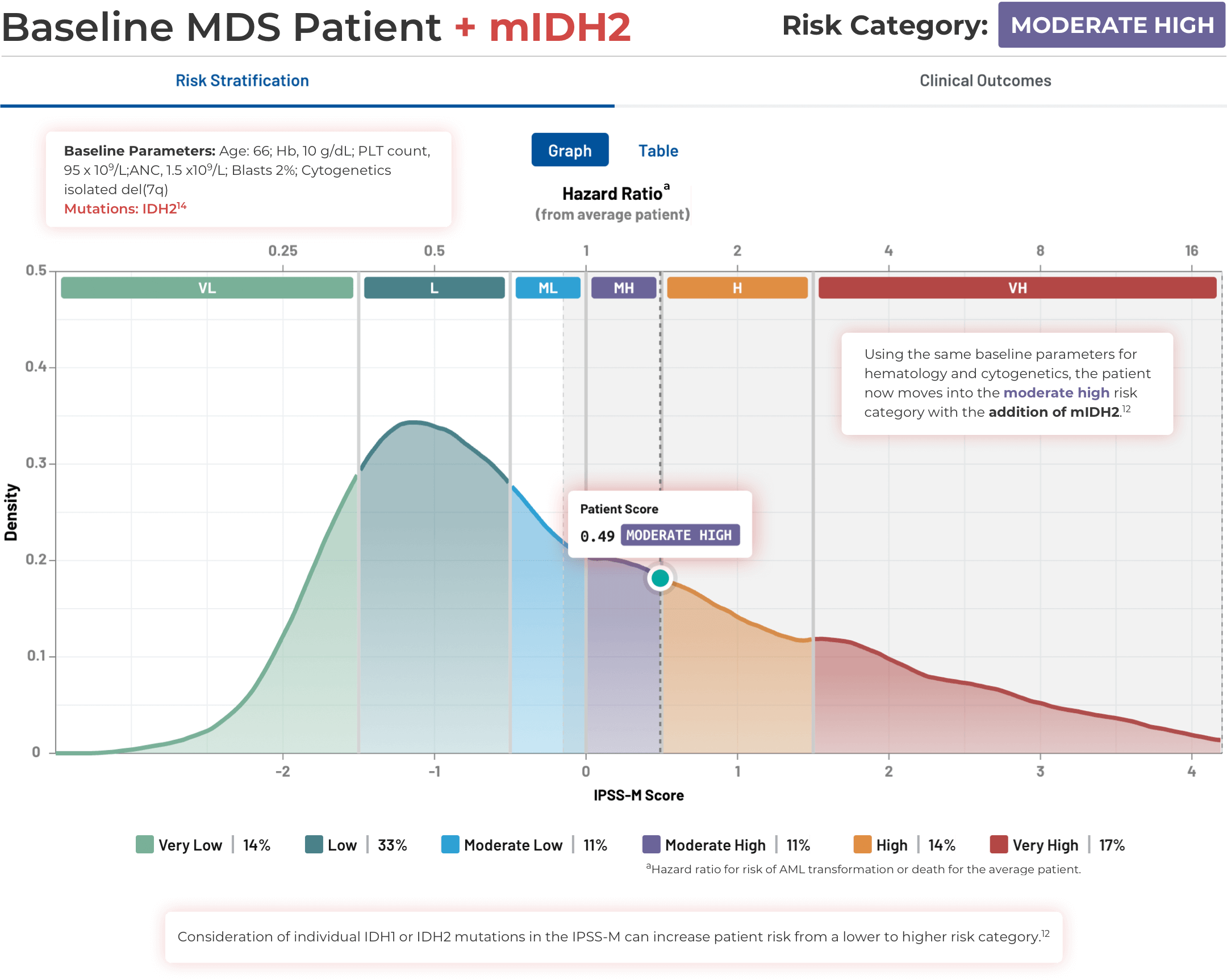
Using the IPSS-M Risk Calculator
Note that IPSS-M results are more uncertain with missing mutation data.11
The Importance of Expedited
Mutational Testing
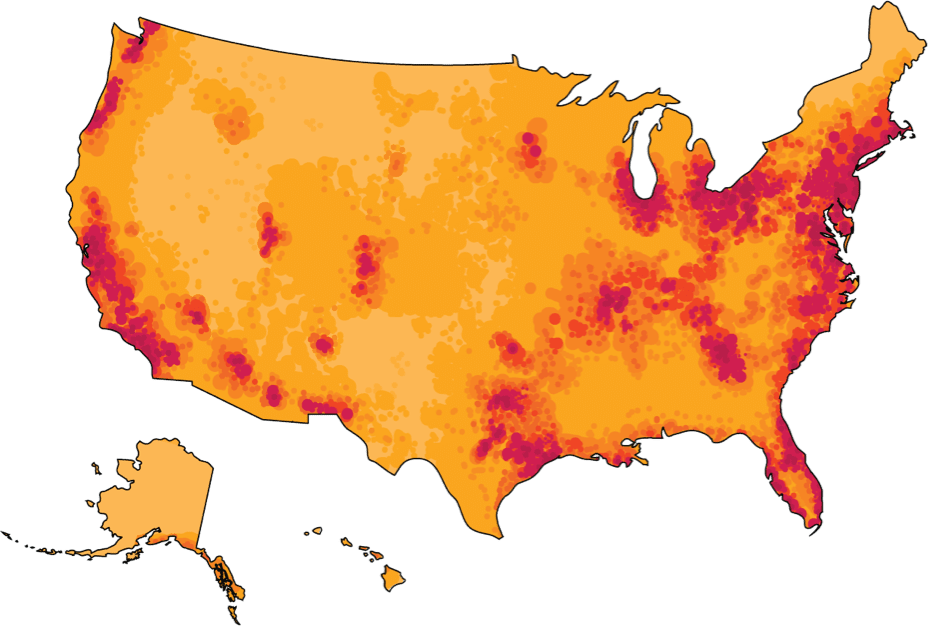
IDH Testing Center Coverage in the
United States*
Recent advances in mutational testing techniques, and availability at commercial reference laboratories, have improved turnaround times in some cases to a few days.16,19-23
Multiple types of assays exist from simple panel tests to full NGS sequencing. Bone marrow aspirate or peripheral blood plasma can be used for mutational testing.16,19 Different methods can be combined to obtain fast answers while waiting for comprehensive results. Consult your local pathologist to discuss ways to optimize sample collection and preservation.16
*Map illustrates general availability of IDH1 panel test across the United States. Servier makes no guarantees to availability of a specific test or testing site in your area. Please contact one of these testing sites directly for additional information.
Stay in the Know
Keep up to date on the latest in
IDH science from leading experts.
aGenerally after no response, intolerance or relapse in lower- and higher-risk MDS.
References:
1. Pirozzi CJ, Yan H. Nat Rev Clin Oncol. 2021;18(10):645-661. doi:10.1038/s41571-021-00521-0 2. Phillips, Carmen. National Cancer Institute Website. https://www.cancer.gov/news-events/cancer-currents-blog/2022/idh1-cancer-metabolite-blocks-immune-cells. Accessed May 12, 2023. 3. RCSB Protein Data Bank website. https://www.rcsb.org/structure/3MAP. Accessed May 18, 2023. 4. Ambinder AJ, DeZern AE. Front Oncol. 2022;12:1033534. doi:10.3389/fonc.2022.1033534 5. Zeidan AM, Pollyea DA, Garcia JS, et al. Blood. 2019;134(Supplement_1):565-565. doi:10.1182/blood-2019-124994 6. Arber DA, Orazi A, Hasserjian RP, et al. Blood. 2022;140(11):1200-1228. doi:10.1182/blood.2022015850 7. Khoury JD, Solary E, Abla O, et al. Leukemia. 2022;36(7):1703-1719. doi:10.1038/s41375-022-01613-1 8. Thol F, Weissinger EM, Krauter J, et al. Haematologica. 2010;95(10):1668-1674. doi:10.3324/haematol.2010.025494 9. Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Leukemia. 2017;31(2):272-281. doi:10.1038/leu.2016.275 10. Jin J, Hu C, Yu M, et al. PLoS ONE. 2014;9(6):e100206. doi:10.1371/journal.pone.0100206 11. Bernard E, Tuechler H, Greenberg PL, et al. NEJM Evidence. 2022;1(7). doi:10.1056/EVIDoa2200008 12. IPSS-M Risk Calculator website. https://mds-risk-model.com/. Accessed March 25, 2023. 13. Aguirre LE, Al Ali N, Sallman DA, et al. Leukemia. Published online May 5, 2023. doi:10.1038/s41375-023-01910-3 14. Cazzola M. Hematology. 2022;2022(1):375-381. doi:10.1182/hematology.2022000349 15. Abbas S, Lugthart S, Kavelaars FG, et al. Blood. 2010;116(12):2122-2126. doi:10.1182/blood-2009-11-250878 16. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed May 20, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 17. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myelodysplastic Syndromes V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed May 20, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 18. Röllig C, Kramer M, Schliemann C, et al. Blood. 2020;136(7):823-830. doi:10.1182/blood.2019004583 19. Baden D, Zukunft S, Hernandez G, et al. Haematologica. 2024;109(8):2469-2477. doi: 10.3324/haematol.2024.285225. 20. Yates SJ, Weiss JJ, Sneider A, et al. Haematologica. 2025 Jul 24. doi: 10.3324/haematol.2025.288085. Online ahead of print.
21. Megías-Vericat JE, Ballesta-López O, Barragán E, Montesinos P. BLCTT. 2019;Volume 9:19-32. doi:10.2147/BLCTT.S177913 22. Duncavage EJ, et al. Blood. 2022;140(21):2228-2247. doi: 10.1182/blood.2022015853 23. Pollyea DA, George TI, Abedi M, et al. eJHaem. 2020;1(1):58-68. doi:10.1002/jha2.16 24. Nelson EJ, et al. Mol Diagn Ther. 2023;27:371-381. doi: 10.1007/s40291-022-00638-7 25. Guijarro F, et al. Curr. Oncol. 2023;30:5201-5213. doi: 10.3390/curroncol30060395
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.